Grow Opportunities
Grow Biotech
Grow Yourself
Save the date: 22-23 April 2026
3rd Global Biologics India
Le Meridien, Hyderabad
22-23 April 2026
Note: Conference will start both days at 9AM
Check in will be open from 8AM
Breakfast would be served from 7 to 9AM

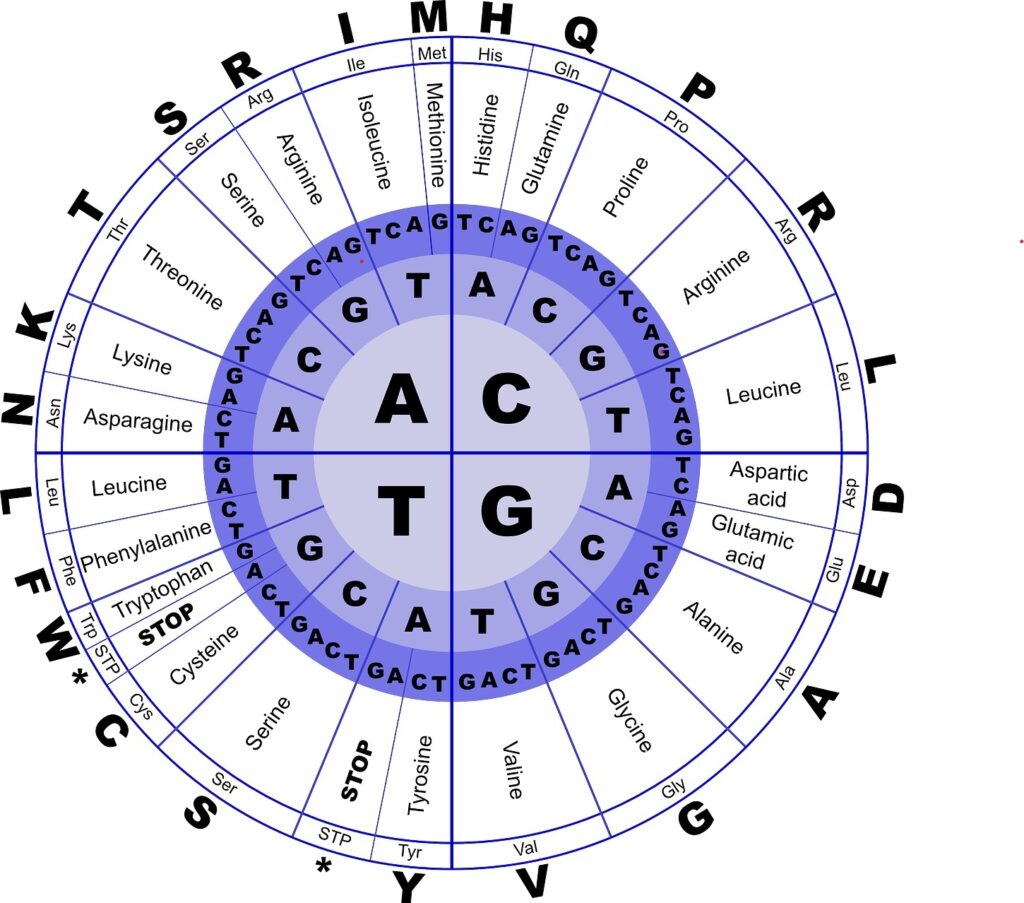
Program Agenda - Modules, Key Topics and Panel Discussion
We will have two tracks on both days: One for Cell and Gene Therapy and other track on Mabs.
Biologics: General Topics
Topic 1: Trends in Biotechnology
Topic 2: AI in Biotech- Endless opportunities
Topic 3: Regulatory and GMP Compliance
Topic 4: Facilities: How India can meet the world’s demand?
Topic 5: Raw Materials: Supply chain and the cost
TRACK 1
Cell and Gene Therapy
Details coming soon...
Panel Discussion 1: Cell and Gene Therapy- Progress, Challenges, and Future Outlook
Panel Members: Dr Dipankar Das, Director and Head, Global Biologics USP, India
Dr Ranjan Chakrabarti, Biopharma and Discovery, Consultant
Dr Renu Kundu, Director and Co Founder, East Ocyon
Dr Arun Anand (Ex COO Immuneel)
Moderator: Coming soon
Panel Discussion 2: Cell and Gene Therapy- New Technologies- CAR T, CRISPR and next….
Panel Members:
Nedunchezhian Murugaiyan, Managing Director, Cytocare; Akhil Kumar, MD, Chief Medical Officer, Aurigene Oncology Ltd
Moderator: Coming soon
Module CGT 1: Cell and Gene Therapy: Challenges and Solutions
Topic 1: Promise of Cell and Gene Therapies: How to overcome barriers for wider adoption of these therapies
Speaker: Raghu Malapaka, Ph.D., Chief Business Officer, Nucelion, India
Topic 2:
Module CGT 2: NK Cells: Practical Solutions
Topic 1: Allogeneic Off-the-Shelf Cell Therapy — Potential, Status and Place in the Indian Landscape
Speaker: Renu Kundu, Ph.D., Director and CoFounder, East Ocyon
Topic 2:
Module CGT 3: CGT: BioManufacturing, Automation at Scale and Bioreactors
Topic 1: Speaker: Vaijainti Gupta, Ph,D., CRisPRbits
Topic 2: Invivo Lentiviral Gene Therapy
Speaker: Sanath Kumar. Team Leader, Microcrispr
Module CGT 4: Stem Cells: Applications and Future Directions
Topic 1: Speaker: Dr Vishwas Kaveeshwar, Head, SDM Uni
Topic 2;
Module CGT 5: Down-Stream purification and GMP Compliance for Cell Therapy products
Topic 1:Opportunities in Downstream Purification and GMP Compliance Challenges for Cell Therapy Products.
Mahesh Bhadane, Head Production Bulk, Advy Chemical
Topic 2
Module CGT 6: Next Generation Cell Therapy products
Topic 1:Reserved for Sponsors
Topic 2
Module CGT 7: AI in CGT: How and what?
Topic 1
Topic 2
Module CGT 8: CAR T cells: How to harness full potential-Next Generation of therapies
Topic 1:The Code is the Cure: Moving from Living Drugs to Injectable CAR Instructions
Dr Murali Addepalli, VP and CSO, Sanshi Bio
Topic 2: CARs, BiTEs, TriTEs, QuTEs… The Power of T cells
Dr Kishore Kunapuli, CSO Cell Therapeutics, Pulse Pharma
Module CGT 9: CGT: Regulatory and GMP Compliance
Topic 1: Comparing Global Regulatory Frameworks for Cell and Gene Therapies and India’s current landscape
Regulatory preparedness for personalised and point of care cell therapies
Speaker: Devesh Panwar, Head Reg Affairrs (CGT), Immuneel, India
Topic 2: Behind the Scenes of Life-Saving Cell and Gene Therapies: Why Quality matters
Pankaj Gupta, Head, QA, KodoLife Sciences
Topic 3: Regulatory Aspects (TBD)
Akhil Kumar, MD, Chief Medical Officer, Aurigene
Module CGT 10: CRISPR/New Technologies
Topic 1: Quality Challenges in CAR-T therapy, CRISPR and iPSC. Speaker: Deepak Sharma, DGM and Head QA, Microcrispr
Module CGT 11: Exosomes in Cell Therapy- New Updates , Development and Global Challenges
Topic 1:
Advancing Cell-Based and Cell-Free Therapeutics: Challenges and the Path to Commercialization
Speaker: Deepika Arora, Ph.D., Lab Director, RCRI StemCell
TRACK 2
Biosimilars and Novel mAbs
Details coming soon...
Panel Discussion 1: mAbs/Biosimilars: Analytics, Regulatory Inspection and GMP Compliance
Panel Members: Prasun Guha, VP Biologics Regulatory Affairs, Dr Reddy Lab; Satish Makkina, GM, Biologics, Procell;
Atin Tomar; Yappan Bio
Dr Ranjan Chakrabarti, Consultant; Dr Sankaranarayanan Srinivasan, CoFounder and Director, Proantek; Nageswara Rao, VP, Reg Affairs, Hetero Drugs;
Moderator: Coming soon
Panel Discussion 2: AI and Biologics: From Bench to Clinical Trials
Panel Members: Prashant Chawla, Sr GM, Manufacturing, Biological Evans
Dr Amit Jogi, VP and Head, Aurigene;
Dhananjay Patankar, Consultant and Former VP, Syngene; Sanjay Shah, Founder, XenPharm; Dr Arpana Dutta, Director/Group Lead, Syngene; Seema Bhandarkar, Head Aurigene
Moderator: Coming soon
Module MAB 1: Upstream and Clone Development
Topic 1: Control Strategy Frameworks and Their Critical Importance in Bioprocessing: Speaker: Harshit Shah, Group Lead, MSAT, Dr Reddys Lab
Topic 2
Module MAB 2:New Tools and Product Development
Topic 1: Best Practices and Tools to Support Consistency of Host Cell Protein Analysis by Mass Spectrometry
Speaker: Dr Sameer Kumar, Manager and Team Lead, Analytics, USP, India
Topic 2: Speaker: Saravanan Desan, Scientific Manager, Biocon
Topic 3: Advanced characterization methods for novel biologics – Now, next or never?
Speaker: Dr Arpana Dutta, Director/Group Lead, Syngene Intl Ltd
Module MAB 3:BioAnalytics: New waves
Topic 1: Speaker: Dr Shubrata Khedkar, Asso director, BioAssay and Mol Biol, USP, India
Topic 2: Speaker: Mansi Jakhade, Analytical Project Manager, Dr Reddys Lab
Topic 1: Next Generation of Biologics
Topic 2
Module MAB 4: Risks and Controls: Manufacturing and Purification
Topic 1: From Risk to Control: Implementing Closed Process Architecture and Contamination Control Strategy in mAbs and bsAbs Downstream Purification
Amit Jogi, Global Head, CDMO Biologics, Aurigene
Topic 2
Module MAB 5: AI- Endless opportunites
Topic 1: AI Transformation for Business Agility in Pharma: Speaker: Seema Bhandarkar
Head Prog and Alliance Mgmt
Aurigene
Topic 2: Reserved for Sponsors
Module MAB 6: Manufacturing- Batch to Continuous mode-What is next?
Topic 1: Batch, Fed‑Batch, and Perfusion Manufacturing Processes in Biopharmaceutical Production Speaker: Mradul Tiwari, Sr Manager, Sun Pharma
Topic 2: Scale-up of biopharma operations in India’s ecosystem; beyond the bioreactor scale-up
Sanjay Shah, Founder, XenPharm Services
Topic 3: Closed Processing – Enabler for NextGen biomanufacturing
Somasundaram G
Principal Consultant, Merck
Module MAB 7: BioAnalytics: New waves
Topic 1: Speaker: Mansi Jakhade, Aaalytical Project Manager, Dr Reddys Lab
Topic 2: Reserved for Sponsors
Module MAB 8: Next Generation Biologics
Topic 1: Reserved
Topic 2: Reserved
Module MAB 9: Bio-Manufacturing/Automation/Digital Tools/Machine Learning
Topic 1: Reserved for sponsors
Topic 2:
Module MAB10: Process Validation, CMC and Regulatory Compliance
Topic 1: GMP and Regulatory Compliance
Mahabubi Shadick, President, Quality Operations, Bharat Biotech
Topic 2: Sweety Mathew, Global RA CMC Lead, Novo Nordisk
Some of the topics/ titles of talks that would be discussed….
Recent Advances in Process Development and Next Generation Manufacturing for Accelerated and Affordable Biosimilar Development
Successful Commercialization of Cell Therapy products in India
Overview of upstream process development for Biosimilars
Successful Cell Therapy Program: challenges associated with drug manufacturing and process and product characterization
Manufacturing of AAV and lentiviral vectors using HEK293 cells
Challenges in process development of CAR T cell therapy
Induced Pluripotent Stem Cells in Regenerative Medicine
NK and Gamma Delta T based Allogenic Cell Therapy
Decoding the future of Healthcare; A comprehensive survey of cell therapy outcomes
Product development: commercialization and market
Regulatory Inspection – Challenges and Opportunities for Biosimilar Products
Regenerative medicine drug development in India: Challenges and Opportunities
Novel Biologics Development- A case study
Strategizing the challenges during clone development
Current challenges of development and cGMP manufacturing of Cell and Gene Therapy products
Induced Pluripotent Stem Cells (iPSC) In Disease Modelling and Drug Discovery
Panel Discussion: Biosimilars: Recent Advances in Cell Engineering and Upstream Processes for Accelerated, high quality and Affordable MAb development
Moderator
Panel Discussion: Cell and Gene Therapy- Progress, Challenges, and future outlook
Moderator
Bioassay: Advancement in functional assessment of biologics and regulatory requirements
Beyond the Blueprint: AI-Powered Protein Engineering for a Brighter Future
Scalable & Efficient Production of Lentiviral vectors
Collaboration of IP and Biosimilar R&D for Early Market Access
Bringing best practices to India in Design of Biopharmaceutical Facilities
Translating Cell and Gene Therapies- From Concept to Commercialization
Agenda from Past Conferences
